-
Pharmaceutical Affairs
Healthcare industry partner
-

Medical Affairs
Healthcare industry partner
Axelys Santé
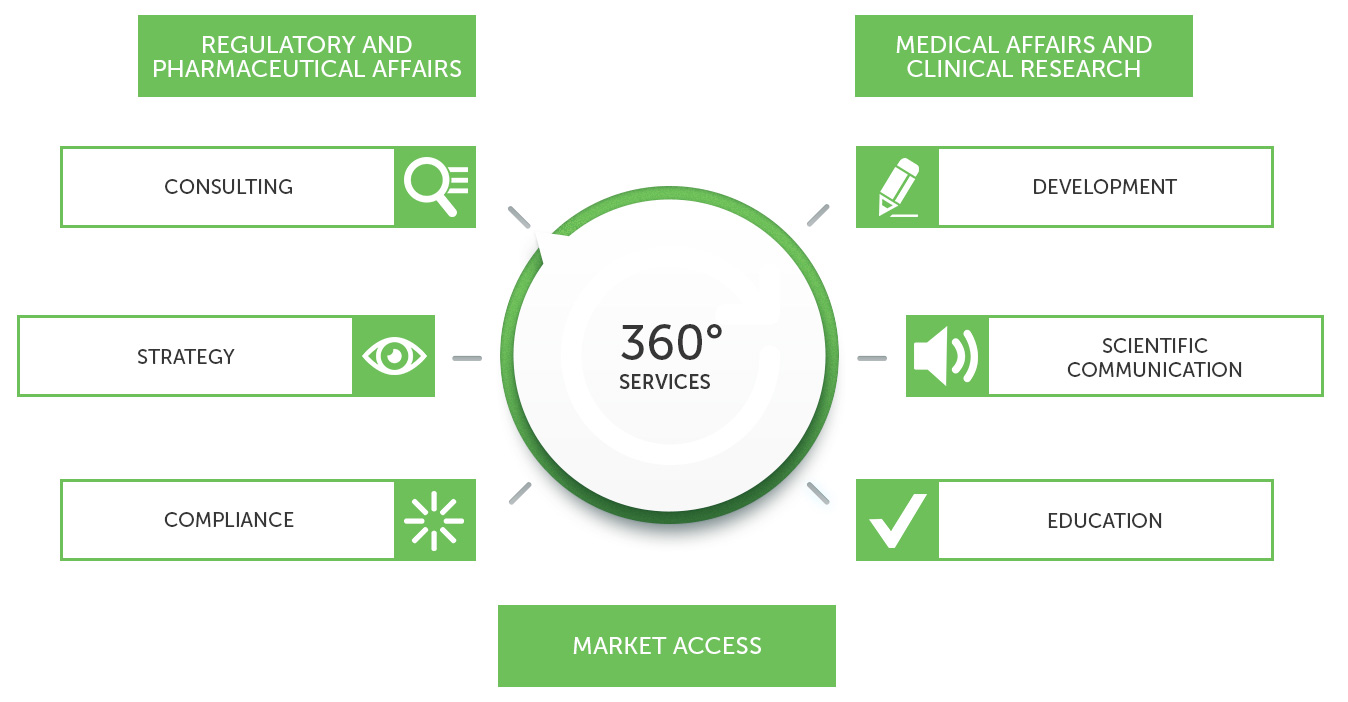
AXELYS SANTE supports healthcare industries and offers a high level of expertise to provide relevant and innovative solutions to issues related to pharmaceutical, medical, economic and regulatory activities.
Our services cover all drugs and other health products: prescription drugs and OTC’s, biotech or gene therapy products, allergens, veterinary medicines, cosmetics and hygiene products, medical devices, food supplements, biocides, ...
Do not hesitate to contact us:
Contact

Dr. Stéphanie NADAL
Regulatory and Pharmaceutical Affairs

Dr. Lamine MAHI
Medical Affairs and Clinical Research
REGULATORY AFFAIRS
We have the knowledge and expertise needed to help you cope with the constant changes in the regulation of health products and to achieve the strategic goals of your organization.
We build for you a customized support, tailored to your needs throughout your product lifecycle.
We work on the following tasks:
- - Control and support in the design of promotional materials and claims
- - Advice and implementation of registration strategies
- - Liaison with Health Authorities: consultation meetings, requests for scientific advice
- - Due diligence
- - European and international regulatory and competitive intelligence
- - Evaluation of the impact of new regulations
- - Support in writing dossiers: CTD’s, labelling, Temporary Recommendations for Use (RTU), …
- - Review of safety data
- - Coaching and training of management teams and regulatory affairs departments on regulatory and economic issues in the field of health in France
×
QUALITY ASSURANCE
We help you to set up, evaluate or optimize your quality management system.
Our expertise allows us to work on the following tasks:
- - Establishment of subsidiaries of pharmaceutical companies in France
- - Pharmaceutical sites files: distributor, manufacturer, importer
- - Establishment and maintenance of your quality system
- - Writing of procedures, instructions, quality manuals
- - Implementation and monitoring of your quality management tools (quality reviews, internal audits, CAPA, ...)
- - Preparation for inspections, training of your teams
- - Support to the realization of audits and inspections
- - Development of indicators to measure your quality of practice or processes
- - Implementation of Medical Visit Charter Code
- - Coaching of “Pharmaciens Responsables”, QP’s and deputies on issues related to their mandates
- - Training of your quality assurance teams
×
MARKET ACCESS
Market Access is an important component of health economics. This is a mandatory step that ensures the access to market of health products with the highest standards of pricing and reimbursement.
Since 2012, regulators alerted about the need to develop health economic studies. It becomes crucial to anticipate and define properly the Price & Reimbursement strategy.
It is also important to position the drug in the treatment pattern where there are clear unmet needs.
Now economic data from real-life studies must be provided for the renewal or maintenance of the price.
We assist companies in the various stages of this process:
- - Market access strategy
- - Pricing and reimbursement (P&R) applications: initial application, renewal
- - Applications for efficiency notice
- - Review of documents
- - Develop and propose add value positioning
- - Validate pricing strategy through Experts advisory boards
×
SCIENTIFIC COMMUNICATION
We offer services within the scope of communication and medical information:
- - Answers to medical questions, providing a scientific reflection, a literature precision and a medical relevance
- - Medical presence when the complexity and the projects specificities required or when there is a request from health professionals
- - Preparation of briefing notes and information at congresses and any other scientific events
- - Medical education for representatives
- - Literature monitoring, updating scientific knowledge
- - Issuing periodic scientific synthesis and summaries
×
MEDICAL AFFAIRS & CLINICAL RESEARCH
We have the scientific and medical knowledge and expertise to assist you in your projects and publications plans. Our experience in clinical development in different therapeutic areas allows us to address different scientific items. We provide real medical expertise in drafting and reviewing your documents.
We can support you in the writing of the following documents:
- - Protocol and Synospsis
- - Clinical Study Report (CSR)
- - Review clinical and clinical summary of the Common Technical Document (CTD)
- - Investigator Brochure
- - Survey or Observational study report
- - Scientific articles and other publications
- - Scientific Communication (newsletter, abstract, poster,...)
Pharmacovigilance: monitoring of activities in clinical trials and post-marketing activities, narrative writing, monitoring and review of literature, monitoring of risk management plans (EU RMP)
Medical support as part of the establishment of Temporary Authorizations for Use (ATU's) and Temporary Recommendations for Use (RTU's)
×
Stéphanie NADAL
Graduated from the Faculty of Pharmacy of Limoges in 1991, Stephanie completed her scientific training with a postgraduate degree at AgroParisTech. began her career at Fresenius, and then worked internationally at Johnson & Johnson in France and Japan in regulatory and marketing functions.
She later became “Pharmacien Responsable – Qualified Person” of Chugai Pharma France, participating in the foundation of the French affiliate of this Japanese company specialized in oncology and biotechnology.
In 2008 she joined Vifor Pharma group where she held the positions of Deputy General Manager and QP of the French affiliate, and Director Regulatory Affairs & Quality Europe, Qualified Person for Pharmacovigilance (EUQP PV).
After completing a certified training in 2013 of administrator at ESSEC business school, she decided to start her consulting company “Axelys Santé Affaires Pharmaceutiques”.
Today, she makes her know-how and her strong international expertise available to companies to assist them in their development, regulatory strategies and the implementation of their quality system.
×
Lamine MAHI
Specialist in Rheumatology and Immunology, Lamine completed his training with a degree in Statistics Applied to Medicine (CESAM) and numerous seminars on Health Management, the last one in 2011 at Harvard School of Public Health. After a short experience in Clinical practices he joined INSERM as Clinical Study Monitor on epidemiological projects in HIV. His career in the industry started with BYK-Gulden as Senior Project Manager in Gastroenterology. Then he joined the LFB (derivated Plasma Products Company) as Head of Immunology Unit in the Medical Department, before working at the international level for Transgene in the planning and the conduct of Clinical Projects in Europe and the USA. In 2002, he joined Gilead Sciences in Paris as Associate Medical Director in anti-infectives, extended later to Compliance, Development and Clinical Operations. In June 2009, he became the Medical Director of Vifor Pharma, contributing to the creation of the French affiliate of the Swiss Pharma Company. He spent 4 years in this position, supervising the medical affairs and market access activities.
In 2013 he decided to create his consulting company Axelys Sante Medical Affairs and Clinical Research, offering his knowledge and expertise for Pharma companies to help them in their clinical development, medical strategies and market access.
×